Homogeneous Catalysis and Biomimetic Synthesis
Asymmetric synthesis is of crucial importance for modern drug discovery. The gap in the “chirality” (fraction of sp3 atoms) of synthesized compounds and accepted drugs is steadily increasing in the last decades, showing the urgent need for the development of novel approaches to chiral organic molecules.
The main goal of our group is to develop novel asymmetric synthetic tools, that will be efficient in terms of enantiomeric excess, regioselectivity and yield. Moreover, we aim to develop sustainable methods that allow researchers to build up molecular complexity in a short number of steps.
In order to address these challenges, our group has two research lines: Asymmetric Catalysis by Transition Metals Complexes and Biomimetic Synthesis of Natural Compounds.

Asymmetric Catalysis by Transition Metals Complexes
Asymmetric catalysis has a very attractive feature of utilizing only limited amounts of precious chiral materials (catalysts) in order to synthesize the bulk of chiral organic products, an approach that is both atom economical and sustainable. Although tremendous progress has been made in asymmetric catalysis in the last decades, the number of industrially applied chiral catalysts is rather limited.
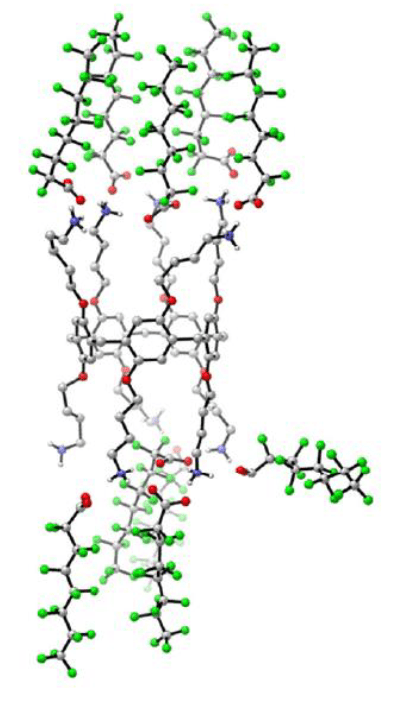
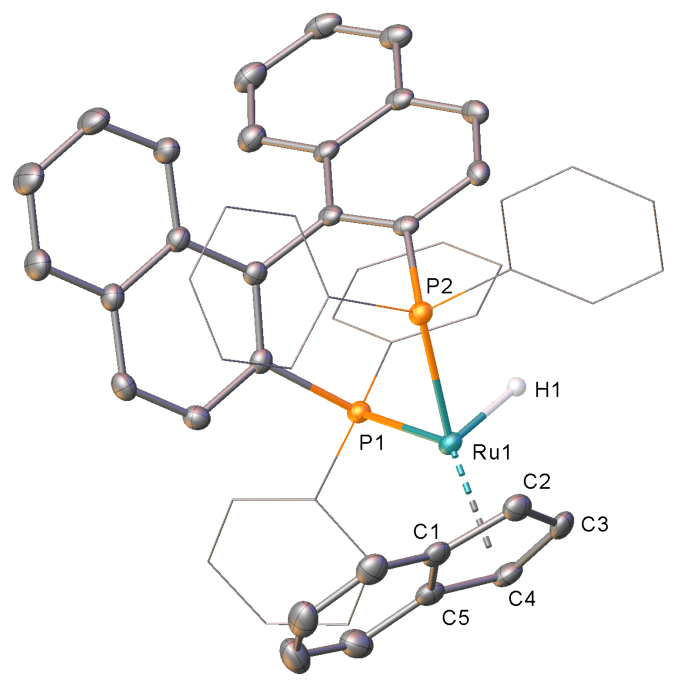
Currently we focus on the development of a new family of chiral Ru(0) complexes for their further application in the fine chemical industry.
Biomimetic Synthesis of Natural Compounds
Natural compounds contain a large number of stereocenters. Realization of their total synthesis in an efficient manner provides natural products and their derivatives for medicinal studies. Total synthesis also benefits the general field of asymmetric synthesis through the development of novel methodologies for introducing, manipulating and multiplying chiral centers within a molecule.